Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review
Abstract
:1. Introduction
1.1. NO Metabolism and Physiological Importance
1.2. The Role of Dietary Nitrates in Exercise Physiology
1.3. Training Status as a Limiting Factor
1.4. Aim and Purpose of the Review
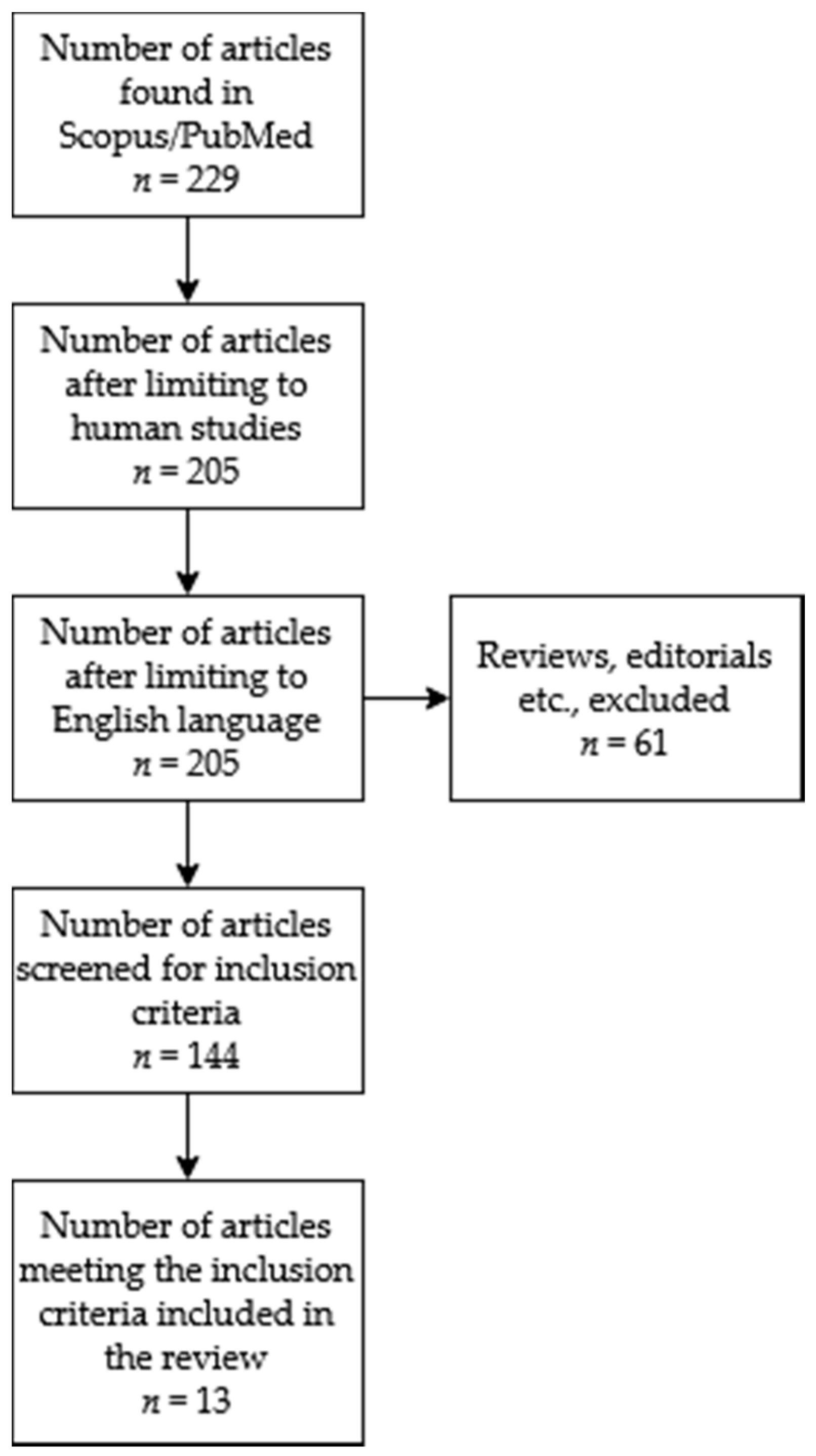
2. Materials and Methods
2.1. Search Strategy, Data Extraction and Analysis
2.2. Data Classification
2.3. Quality Assessment
2.4. Data Extraction
2.5. Statistical Analysis
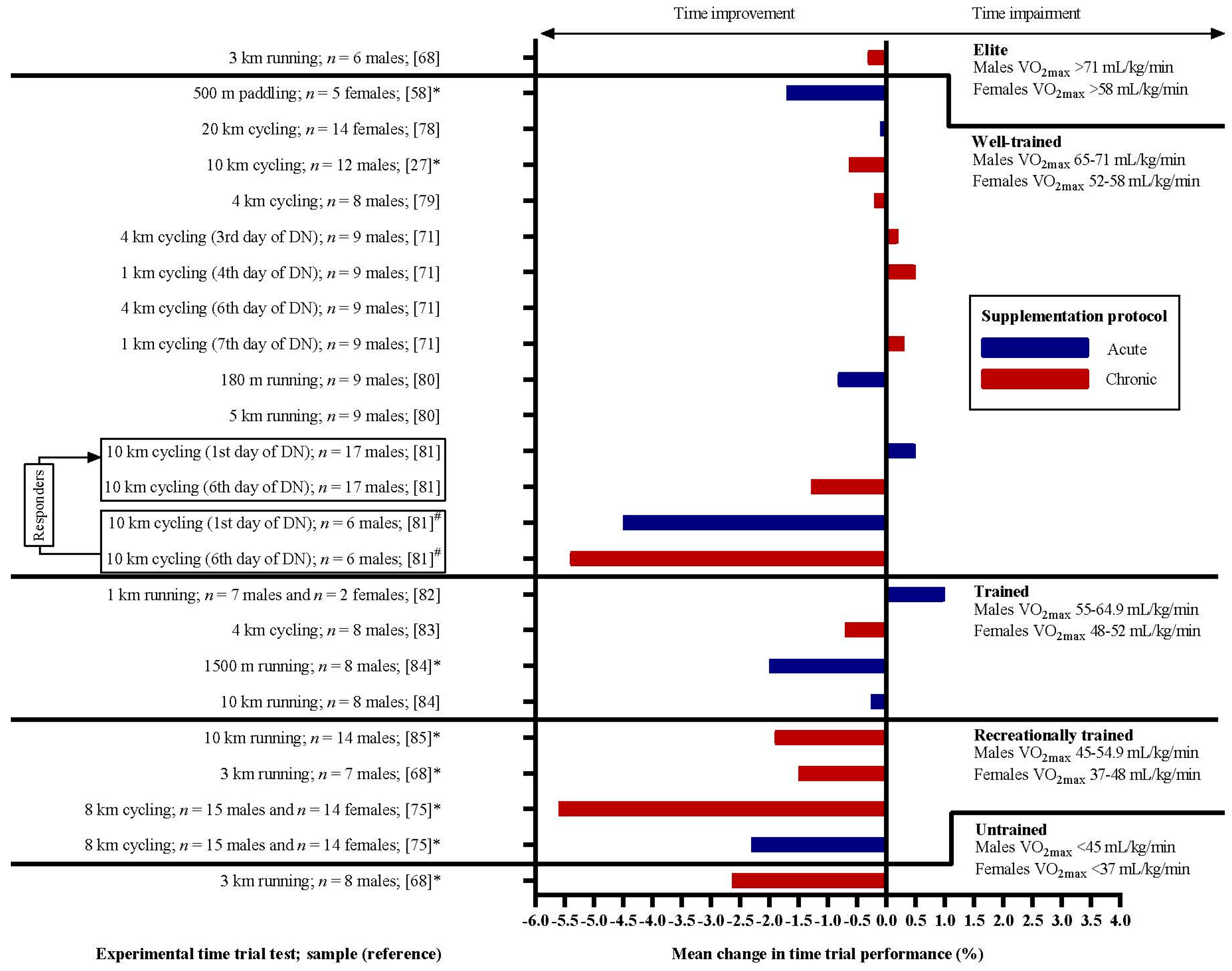
3. Results
Methodological Quality of Studies
4. Discussion
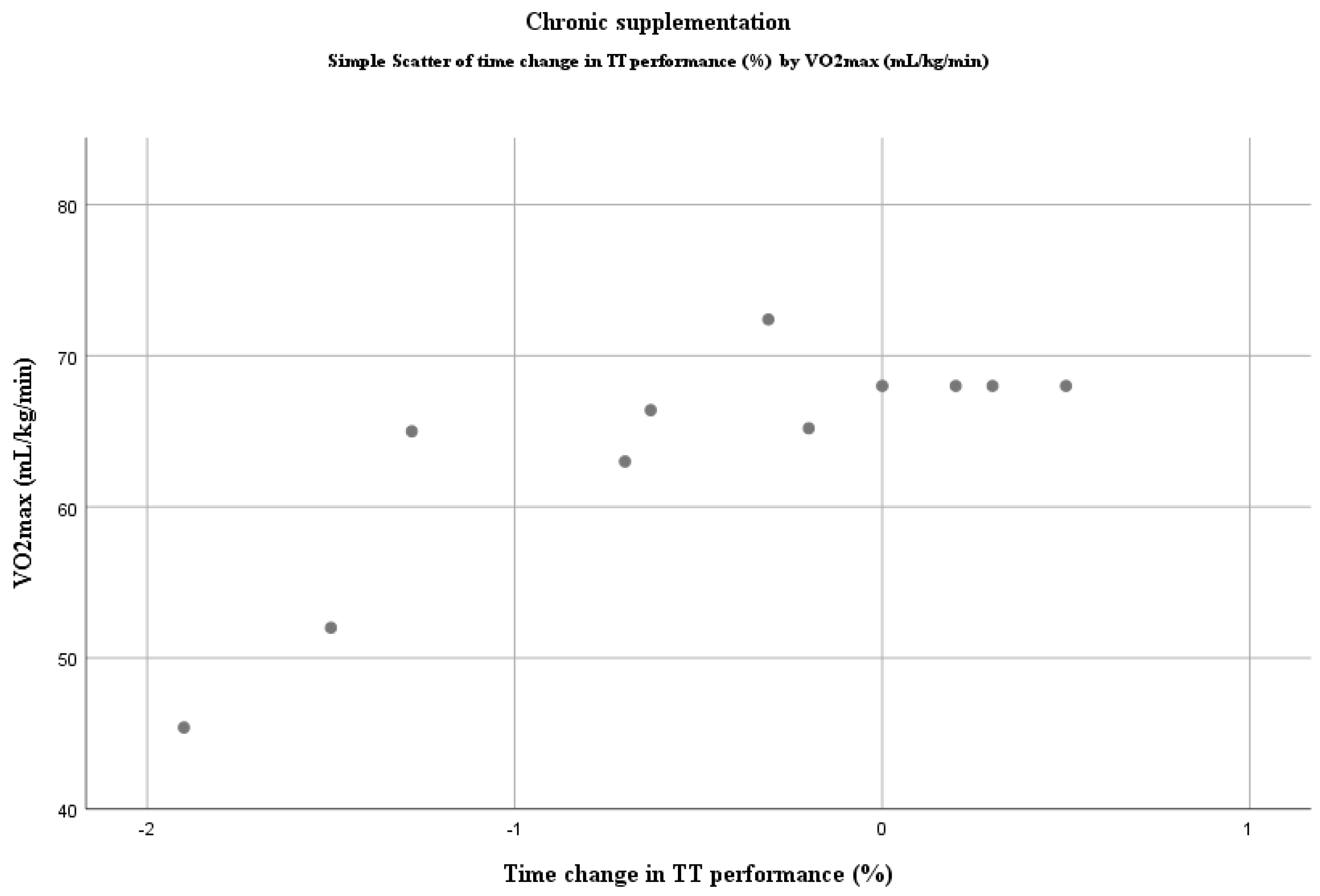
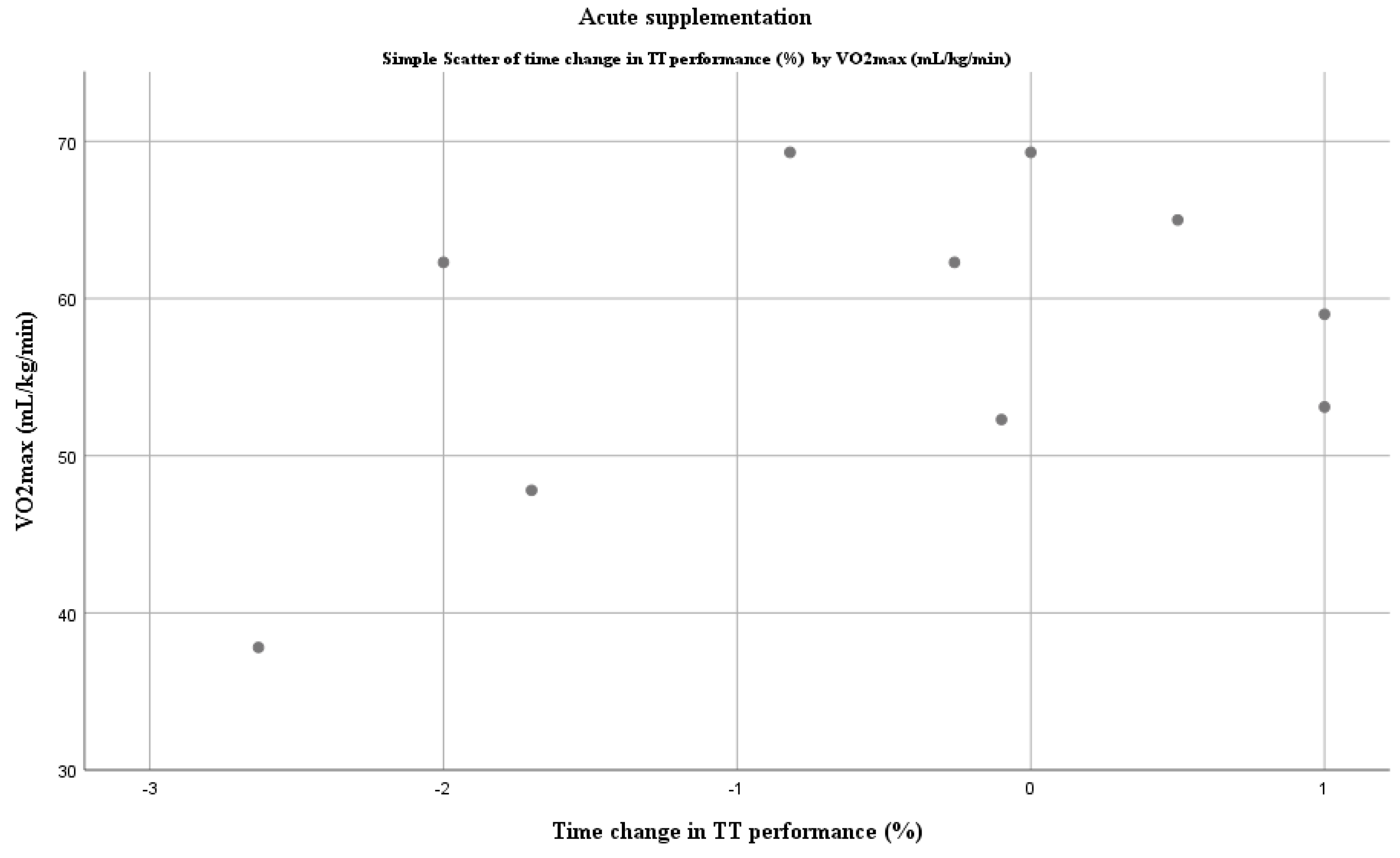
4.1. Dietary Nitrates and Time Trial Performance
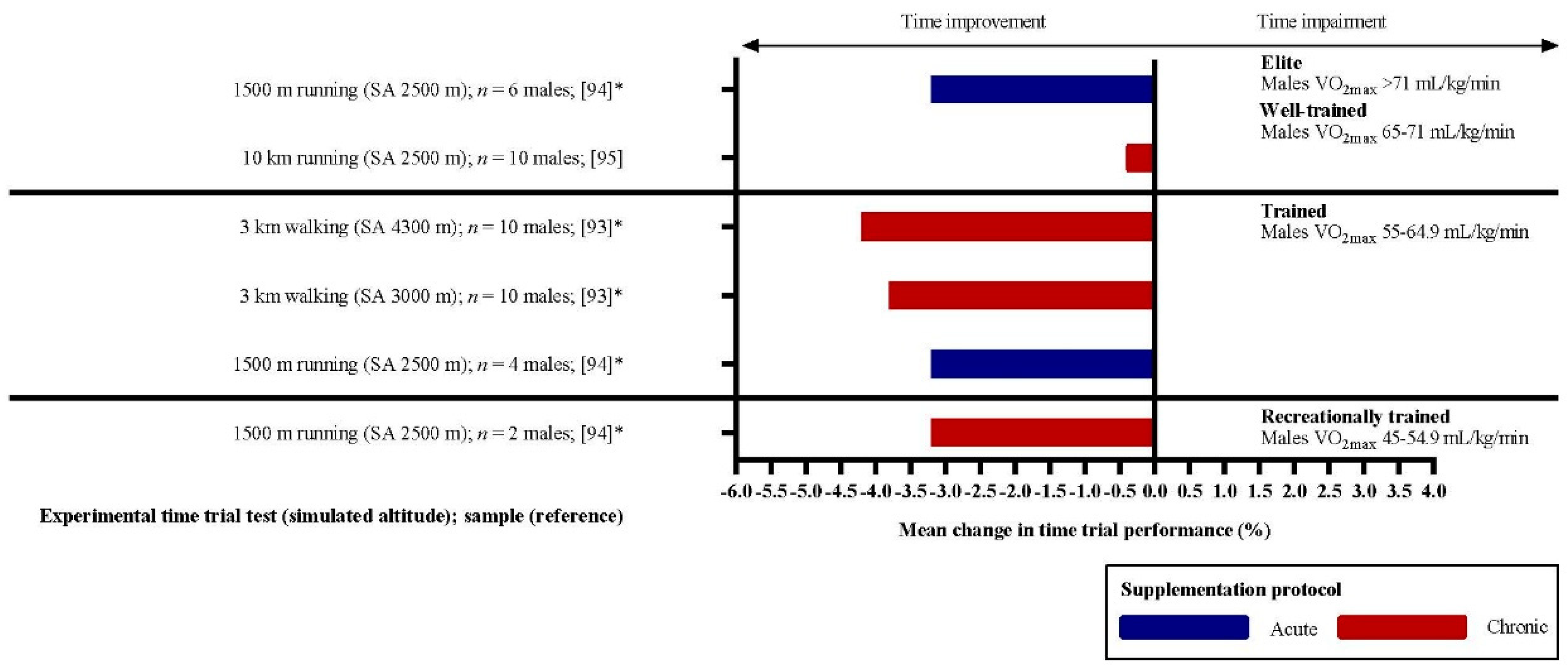
4.2. Dietary Nitrates and Exercise Performance in a High-Altitude Environment
4.3. Dietary Nitrates Studies Methodology
4.4. The Real Benefit for Elite Athletes?
4.5. Future Perspectives
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef]
- Murad, F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 1999, 19, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J. Nitric oxide: A unique endogenous signaling molecule in vascular biology. Biosci. Rep. 1999, 19, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F. Endothelium-derived relaxing factor: Discovery, early studies, and identification as nitric oxide. Biosci. Rep. 1999, 19, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Howatson, G.; West, D.J.; Stevenson, E.J. The potential benefits of red beetroot supplementation in health and disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef]
- Burke, L.M. Practical issues in evidence-based use of performance supplements: Supplement interactions, repeated use and individual responses. Sports Med. 2017, 47, 79–100. [Google Scholar] [CrossRef] [Green Version]
- Peeling, P.; Castell, L.M.; Derave, W.; de Hon, O.; Burke, L.M. Sports foods and dietary supplements for optimal function and performance enhancement in track-and-field athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Vitale, K.; Getzin, A. Nutrition and supplement update for the endurance athlete: Review and recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [Green Version]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiologic context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; van Loon, L.J.C.; Verdijk, L.B. Can elite athletes benefit from dietary nitrate supplementation? J. Appl. Physiol. 2015, 119, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Murad, F. Nitric oxide and cyclic GMP in cell signaling and drug development. N. Engl. J. Med. 2006, 355, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, P.M.; Leone, A.M.; Francis, P.L.; Struthers, A.D.; Moncada, S. The L-arginine: Nitric oxide pathway is the major source of plasma nitrite in fasted humans. Biochem. Biophys. Res. Commun. 1995, 209, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Carlström, M.; Larsen, F.J.; Weitzberg, E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide 2014, 38, 45–57. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546. [Google Scholar] [CrossRef]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- James, P.E.; Willis, G.R.; Allen, J.D.; Winyard, P.G.; Jones, A.M. Nitrate pharmacokinetics: Taking note of the difference. Nitric Oxide 2015, 48, 44–50. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch. Pharm. Res. 2009, 32, 1119–1126. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [Green Version]
- Moncada, S.; Higgs, A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarti, P.; Forte, E.; Mastronicola, D.; Giuffrè, A.; Arese, M. Cytochrome c oxidase and nitric oxide in action: Molecular mechanisms and pathophysiological implications. Biochim. Biophys. Acta 2012, 1817, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Van Faassen, E.E.; Bahrami, S.; Feelisch, M.; Hogg, N.; Kelm, M.; Kim-Shapiro, D.B.; Kozlov, A.V.; Li, H.; Lundberg, J.O.; Mason, R.; et al. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med. Res. Rev. 2009, 29, 683–741. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [Green Version]
- Coggan, A.R.; Peterson, L.R. Dietary nitrate enhances the contractile properties of human skeletal muscle. Exerc. Sport Sci. Rev. 2018, 46, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Rokkedal-Lausch, T.; Franch, J.; Poulsen, M.K.; Thomsen, L.P.; Weitzberg, E.; Kamavuako, E.N.; Karbing, D.S.; Larsen, R.G. Chronic high-dose beetroot juice supplementation improves time trial performance of well-trained cyclists in normoxia and hypoxia. Nitric Oxide Biol. Chem. 2019, 85, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Hoon, M.W.; Jones, A.M.; Johnson, N.A.; Blackwell, J.R.; Broad, E.M.; Lundy, B.; Rice, A.J.; Burke, L.M. The effect of variable doses of inorganic nitrate-rich beetroot juice on simulated 2,000-m rowing performance in trained athletes. Int. J. Sports Physiol. Perform. 2014, 9, 615–620. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [Green Version]
- Wylie, L.J.; Ortiz de Zevallos, J.; Isidore, T.; Nyman, L.; Vanhatalo, A.; Bailey, S.J.; Jones, A.M. Dose-dependent effects of dietary nitrate on the oxygen cost of moderate-intensity exercise: Acute vs. chronic supplementation. Nitric Oxide 2016, 57, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, K.G.; Turner, L.; Prichard, J.; Dodd, F.; Kennedy, D.O.; Haskell, C.; Blackwell, J.R.; Jones, A.M. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir. Physiol. Neurobiol. 2014, 193, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Muggeridge, D.J.; Sculthorpe, N.; Grace, F.M.; Willis, G.; Thornhill, L.; Weller, R.B.; James, P.E.; Easton, C. Acute whole body UVA irradiation combined with nitrate ingestion enhances time trial performance in trained cyclists. Nitric Oxide 2015, 48, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Folland, J.P. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, J.; Gamu, D.; Heigenhauser, G.J.F.; van Loon, L.J.C.; Spriet, L.L.; Tupling, A.R.; Holloway, G.P. Beetroot juice increases human muscle force without changing Ca2+-handling proteins. Med. Sci. Sports Exerc. 2017, 49, 2016–2024. [Google Scholar] [CrossRef]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber Type-Specific Effects of Dietary Nitrate. Exerc. Sport Sci. Rev. 2016, 44, 53. [Google Scholar] [CrossRef]
- Sussman, I.; Erecińska, M.; Wilson, D.F. Regulation of cellular energy metabolism. The Crabtree effect. Biochim. Biophys. Acta Bioenerg. 1980, 591, 209–223. [Google Scholar] [CrossRef]
- Nourry, C.; Fabre, C.; Bart, F.; Grosbois, J.-M.; Berthoin, S.; Mucci, P. Evidence of exercise-induced arterial hypoxemia in prepubescent trained children. Pediatr. Res. 2004, 55, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E. NO-synthase independent NO generation in mammals. Biochem. Biophys. Res. Commun. 2010, 396, 39–45. [Google Scholar] [CrossRef]
- Modin, A.; Björne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of “acidic-metabolic” vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Behnke, B.J.; McDonough, P.; Padilla, D.J.; Musch, T.I.; Poole, D.C. Oxygen exchange profile in rat muscles of contrasting fibre types. J. Physiol. 2003, 549, 597–605. [Google Scholar] [CrossRef] [PubMed]
- McDonough, P.; Behnke, B.J.; Padilla, D.J.; Musch, T.I.; Poole, D.C. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J. Physiol. 2005, 563, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; McDonough, P.; Behnke, B.J.; Musch, T.I.; Poole, D.C. Blood flow and O2 extraction as a function of O2 uptake in muscles composed of different fiber types. Respir. Physiol. Neurobiol. 2006, 153, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F.; Bevers, L.M.; Slama-Schwok, A.; van Faassen, E.E. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell. Mol. Life Sci. 2007, 64, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Varnham, R.L.; DiMenna, F.J.; Breese, B.C.; Wylie, L.J.; Jones, A.M. Inorganic nitrate supplementation improves muscle oxygenation, O₂ uptake kinetics, and exercise tolerance at high but not low pedal rates. J. Appl. Physiol. 2015, 118, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J.C. No improvement in endurance performance after a single dose of beetroot juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of nitrate supplementation on VO₂ kinetics and endurance of elite cyclists. Scand. J. Med. Sci. Sports 2013, 23, e21–e31. [Google Scholar] [CrossRef]
- Mosher, S.L.; Gough, L.A.; Deb, S.; Saunders, B.; Naughton, L.R.M.; Brown, D.R.; Sparks, S.A. High dose Nitrate ingestion does not improve 40 km cycling time trial performance in trained cyclists. Res. Sports Med. 2020, 28, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, R.K.; Whitfield, J.; Spriet, L.L. Beetroot juice supplementation does not improve performance of elite 1500-m runners. Med. Sci. Sports Exerc. 2014, 46, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
- Bescós, R.; Ferrer-Roca, V.; Galilea, P.A.; Roig, A.; Drobnic, F.; Sureda, A.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med. Sci. Sports Exerc. 2012, 44, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Peacock, O.; Tjønna, A.E.; James, P.; Wisløff, U.; Welde, B.; Böhlke, N.; Smith, A.; Stokes, K.; Cook, C.; Sandbakk, Ø. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med. Sci. Sports Exerc. 2012, 44, 2213–2219. [Google Scholar] [CrossRef]
- Coyle, E.F.; Feltner, M.E.; Kautz, S.A.; Hamilton, M.T.; Montain, S.J.; Baylor, A.M.; Abraham, L.D.; Petrek, G.W. Physiological and biomechanical factors associated with elite endurance cycling performance. Med. Sci. Sports Exerc. 1991, 23, 93–107. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Craig, N.P.; Hawley, J.A. The bioenergetics of world class cycling. J. Sci. Med. Sport 2000, 3, 414–433. [Google Scholar] [CrossRef]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2010, 110, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Peeling, P.; Cox, G.R.; Bullock, N.; Burke, L.M. Beetroot juice improves on-water 500 M time-trial performance, and laboratory-based paddling economy in national and international-level kayak athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 278–284. [Google Scholar] [CrossRef]
- Muggeridge, D.J.; Howe, C.C.F.; Spendiff, O.; Pedlar, C.; James, P.E.; Easton, C. The effects of a single dose of concentrated beetroot juice on performance in trained flatwater kayakers. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 498–506. [Google Scholar] [CrossRef]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Polgar, J.; Johnson, M.A.; Weightman, D.; Appleton, D. Data on fibre size in thirty-six human muscles: An autopsy study. J. Neurol. Sci. 1973, 19, 307–318. [Google Scholar] [CrossRef]
- Jennekens, F.G.I.; Tomlinson, B.E.; Walton, J.N. The sizes of the two main histochemical fibre types in five limb muscles in man: An autopsy study. J. Neurol. Sci. 1971, 13, 281–292. [Google Scholar] [CrossRef]
- Roth, W.; Schwanitz, P.; Pas, P.; Bauer, P. Force-time characteristics of the rowing stroke and corresponding physiological muscle adaptations. Int. J. Sports Med. 1993, 14, S32–S34. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Science and medicine of canoeing and kayaking. Sports Med. 1987, 4, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, J. Physiological aspects of training in rowing. Int. J. Sports Med. 1993, 14 (Suppl. 1), S3. [Google Scholar]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef] [Green Version]
- Bryan, N.S.; Ivy, J.L. Inorganic nitrite and nitrate: Evidence to support consideration as dietary nutrients. Nutr. Res. 2015, 35, 643–654. [Google Scholar] [CrossRef]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Rasica, L.; Marzorati, M. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Tesch, P.A.; Karlsson, J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J. Appl. Physiol. 1985, 59, 1716–1720. [Google Scholar] [CrossRef]
- Proctor, D.N.; Sinning, W.E.; Walro, J.M.; Sieck, G.C.; Lemon, P.W. Oxidative capacity of human muscle fiber types: Effects of age and training status. J. Appl. Physiol. 1995, 78, 2033–2038. [Google Scholar] [CrossRef]
- McQuillan, J.A.; Dulson, D.K.; Laursen, P.B.; Kilding, A.E. Dietary nitrate fails to improve 1 and 4 km cycling performance in highly trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; De Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to classify subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decroix, L.; Pauw, K.D.; Foster, C.; Meeusen, R. Guidelines to classify female subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2016, 11, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Fischer, M.; Auslander, A.T.; Beigarten, A.; Daggy, B.; Hansen, K.; Kessler, L.; Osmond, A.; Wang, H.; Wes, R. The effects of multi-day vs. single pre-exercise nitrate supplement dosing on simulated cycling time trial performance and skeletal muscle oxygenation. J. Strength Cond. Res. 2019, 33, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Glaister, M.; Pattison, J.R.; Muniz-Pumares, D.; Patterson, S.D.; Foley, P. Effects of dietary nitrate, caffeine, and their combination on 20-km cycling time trial performance. J. Strength Cond. Res. 2015, 29, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Callahan, M.J.; Parr, E.B.; Hawley, J.A.; Burke, L.M. Single and combined effects of beetroot crystals and sodium bicarbonate on 4-km cycling time trial performance. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 271–278. [Google Scholar] [CrossRef]
- Sandbakk, S.B.; Sandbakk, Ø.; Peacock, O.; James, P.; Welde, B.; Stokes, K.; Böhlke, N.; Tjønna, A.E. Effects of acute supplementation of L-arginine and nitrate on endurance and sprint performance in elite athletes. Nitric Oxide Biol. Chem. 2015, 48, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Nyakayiru, J.M.; Jonvik, K.L.; Pinckaers, P.J.M.; Senden, J.; van Loon, L.J.C.; Verdijk, L.B. No effect of acute and 6-day nitrate supplementation on VO2 and time-trial performance in highly trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, J.; McGawley, K. No individual or combined effects of caffeine and beetrootjuice supplementation during submaximal or maximal running. Appl. Physiol. Nutr. Metab. 2018, 43, 697–703. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, J.A.; Dulson, D.K.; Laursen, P.B.; Kilding, A.E. The effect of dietary nitrate supplementation on physiology and performance in trained cyclists. Int. J. Sports Physiol. Perform. 2017, 12, 684–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, O.M.; Barlow, M.J.; Duckworth, L.; Williams, E.; Wort, G.; Woods, D.; Siervo, M.; O’Hara, J.P. Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur. J. Appl. Physiol. 2017, 117, 775–785. [Google Scholar] [CrossRef]
- de Castro, T.F.; Manoel, F.A.; Figueiredo, D.H.; Figueiredo, D.H.; Machado, F.A. Effect of beetroot juice supplementation on 10-km performance in recreational runners. Appl. Physiol. Nutr. Metab. 2019, 44, 90–94. [Google Scholar] [CrossRef] [Green Version]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: A systematic review and meta-analysis. Sports Med. 2017, 47, 735–756. [Google Scholar] [CrossRef] [Green Version]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [Green Version]
- Nyakayiru, J.; van Loon, L.C.; Verdijk, L. Could intramuscular storage of dietary nitrate contribute to its ergogenic effect? A mini-review. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef]
- Murray, A.J.; Horscroft, J.A. Mitochondrial function at extreme high altitude. J. Physiol. 2016, 594, 1137–1149. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.J. Metabolic adaptation of skeletal muscle to high altitude hypoxia: How new technologies could resolve the controversies. Genome Med. 2009, 1, 117. [Google Scholar] [CrossRef]
- Kelly, J.; Vanhatalo, A.; Bailey, S.J.; Wylie, L.J.; Tucker, C.; List, S.; Winyard, P.G.; Jones, A.M. Dietary nitrate supplementation: Effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R920–R930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masschelein, E.; van Thienen, R.; Wang, X.; van Schepdael, A.; Thomis, M.; Hespel, P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 2012, 113, 736–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Deighton, K.; Matu, J.; Williams, E.L.; Woods, D.; Xie, L.; Stephan, B.C.M.; Siervo, M.; et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Woods, D.; Lara, J.; Siervo, M.; O’Hara, J.P. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide Biol. Chem. 2016, 59, 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, J.T.; Oliver, S.J.; Lewis-Jones, T.M.; Wylie, L.J.; Macdonald, J.H. Beetroot juice does not enhance altitude running performance in well-trained athletes. Appl. Physiol. Nutr. Metab. 2015, 40, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Puype, J.; Ramaekers, M.; van Thienen, R.; Deldicque, L.; Hespel, P. No effect of dietary nitrate supplementation on endurance training in hypoxia. Scand. J. Med. Sci. Sports 2015, 25, 234–241. [Google Scholar] [CrossRef]
- Cumpstey, A.F.; Hennis, P.J.; Gilbert-Kawai, E.T.; Fernandez, B.O.; Grant, D.; Jenner, W.; Poudevigne, M.; Moyses, H.; Levett, D.Z.; Cobb, A.; et al. Effects of dietary nitrate supplementation on microvascular physiology at 4559 m altitude—A randomised controlled trial (Xtreme Alps). Nitric Oxide 2020, 94, 27–35. [Google Scholar] [CrossRef]
- Cumpstey, A.F.; Hennis, P.J.; Gilbert-Kawai, E.T.; Fernandez, B.O.; Poudevigne, M.; Cobb, A.; Meale, P.; Mitchell, K.; Moyses, H.; Pöhnl, H.; et al. Effects of dietary nitrate on respiratory physiology at high altitude—Results from the Xtreme Alps study. Nitric Oxide 2017, 71, 57–68. [Google Scholar] [CrossRef]
- Bakker, E.; Engan, H.; Patrician, A.; Schagatay, E.; Karlsen, T.; Wisløff, U.; Gaustad, S.E. Acute dietary nitrate supplementation improves arterial endothelial function at high altitude: A double-blinded randomized controlled cross over study. Nitric Oxide 2015, 50, 58–64. [Google Scholar] [CrossRef]
- Hultström, M. Commentaries on Viewpoint: Can elite athletes benefit from dietary nitrate supplementation? J. Appl. Physiol. 2015, 119, 762–769. [Google Scholar] [CrossRef]
- Giro d’Italia 2019 | Stage 21 (ITT) | Results. Available online: https://www.procyclingstats.com/race/giro-d-italia/2019/stage-21 (accessed on 10 May 2020).
- Tour de France 2018 | Stage 20 (ITT) | Results. Available online: https://www.procyclingstats.com/race/tour-de-france/2018/stage-20 (accessed on 10 May 2020).
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, G.; Nevill, A.M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med. 1998, 26, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Paton, C.D.; Hopkins, W.G. Tests of cycling performance. Sports Med. 2001, 31, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Kasper, A.M.; Morton, J.P. From paper to podium: Quantifying the translational potential of performance nutrition research. Sports Med. 2019, 49, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Piknova, B.; Park, J.W.; Lam, K.K.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric Oxide 2016, 55, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Gilliard, C.N.; Lam, J.K.; Cassel, K.S.; Park, J.W.; Schechter, A.N.; Piknova, B. Effect of dietary nitrate levels on nitrate fluxes in rat skeletal muscle and liver. Nitric Oxide 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Kapur, S.; Bédard, S.; Marcotte, B.; Côté, C.H.; Marette, A. Expression of nitric oxide synthase in skeletal muscle: A novel role for nitric oxide as a modulator of insulin action. Diabetes 1997, 46, 1691–1700. [Google Scholar] [CrossRef]
- Nyakayiru, J.; Kouw, I.W.K.; Cermak, N.M.; Senden, J.M.; van Loon, L.J.C.; Verdijk, L.B. Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J. Appl. Physiol. 2017, 123, 637–644. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, S.T.J.; Wylie, L.J.; Webster, J.M.A.; Vanhatalo, A.; Jones, A.M. Influence of dietary nitrate food forms on nitrate metabolism and blood pressure in healthy normotensive adults. Nitric Oxide 2018, 72, 66–74. [Google Scholar] [CrossRef] [PubMed]
| Training Status (Number of Experimental Situations Included) | Sex | VO2max (mL/kg/min) |
|---|---|---|
| 1—Untrained (n = 1) | Males | <45 |
| Females | <37 | |
| 2—Recreationally trained (n = 4) | Males | 45–54.9 |
| Females | 37–48 | |
| 3—Trained (n = 4) | Males | 55–64.9 |
| Females | 48–52 | |
| 4—Well-trained (n = 12) | Males | 65–71 |
| Females | 52–58 | |
| 5—Elite (n = 1) | Males | >71 |
| Females | >58 |
| Reference | Sex | Training Status | VO2max a/VO2peak b | CV | A/C | Supplementation Protocol | Test (Test Duration) | Ergogenic * |
|---|---|---|---|---|---|---|---|---|
| [75] | M and F | Recreationally trained | - | - | Acute | 8.0 mmol (NO3−) of PN; 2 h before the test | 8 km cycling (~20 min) | Yes |
| [84] | M | Trained | 62.3 ± 8.1 mL/kg/min a | 13.0% | 12.8 mmol (NO3−) of BJC; 3 h before the test | 1500 m running (~6 min) | Yes | |
| 10 km running (~45 min) | No | |||||||
| [82] | M | Trained | 59.0 ± 2.9 mL/kg/min a | 4.9% | 6.4 mmol (NO3−) of BJC; 2.5 h before the test | 1 km running (<3 min) | No | |
| F | 53.1 ± 11.4 mL/kg/min a | 21.5% | ||||||
| [80] | M | Well-trained | 69.3 ± 5.8 mL/kg/min a | 8.4% | 9.9 mmol (NO3−) of PN; 2.5 h before the test | 180 m running (~25 s) | No | |
| 5 km running (~17 min) | No | |||||||
| [81] | M | Well-trained | 65.0 ± 4.0 mL/kg/min b | 6.2% | 12.8 mmol (NO3−) of SN; 3 h before the test | 10 km cycling (~17 min) | No # | |
| [78] | F | Well-trained | 52.3 ± 4.9 mL/kg/min a | 9.4% | 6.4 mmol (NO3−) of BJC; 2.5 h before the test | 20 km cycling (~35 min) | No | |
| [58] | F | Well-trained † | 47.8 ± 3.7 mL/kg/min b | 7.7% | 12.8 mmol NO3−) of BJC; 2 h before the test | 500 m paddling (~2 min) | Yes | |
| [68] | M | Untrained | 37.8 ± 5.8 mL/kg/min b | 15.3% | Chronic | 6-day 5.5 mmol (NO3−) of SN; 3.5 h before the test | 3 km running (~15 min) | Yes |
| [75] | M and F | Recreationally trained | - | - | 15-day 8.0 mmol (NO3−) of PN; 2 h before the test | 8 km cycling (~20 min) | Yes | |
| [68] | M | Recreationally trained | 52.0 ± 4.5 mL/kg/min b | 8.7% | 6-day 5.5 mmol (NO3−) of SN; 3.5 h before the test | 3 km running (~12 min) | Yes | |
| [85] | M | Recreationally trained | 45.4 ± 5.9 mL/kg/min a | 13.0% | 3-day 8.4 mmol (NO3−) of BJ; 2 h before the test | 10 km running (~55 min) | Yes | |
| [83] | M | Trained | 63.0 ± 4.0 mL/kg/min b | 6.3% | 8-day 6.4 mmol (NO3−) of BJC; 2.5 h before the test | 4 km cycling (~6 min) | No | |
| [71] | M | Well-trained | 68.0 ± 3.0 mL/kg/min b | 4.4% | 7-day 12.8 mmol (NO3−) of BJC; 2.5 h before the test | 1 km cycling (~80 s) | No | |
| 4 km cycling (<6 min) | No | |||||||
| [79] | M | Well-trained | 65.2 ± 4.2 mL/kg/min a | 6.4% | 3-day ~5 mmol (NO3−) of BC; 1 h before the test | 4 km cycling (<6 min) | No | |
| [81] | M | Well-trained | 65.0 ± 4.0 mL/kg/min b | 6.2% | 6-day 12.8 mmol (NO3−) of SN; 3 h before the test | 10 km cycling (~17 min) | No # | |
| [27] | M | Well-trained | 66.4 ± 5.3 mL/kg/min a | 8.0% | 7-day 12.8 mmol (NO3−) of BJC; 2.75 h before the test | 10 km cycling (~15 min) | Yes | |
| [68] | M | Elite | 72.4 ± 6.1 mL/kg/min b | 8.4% | 6-day 5.5 mmol (NO3−) of SN; 3.5 h before the test | 3 km running (~10 min) | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlinský, T.; Kumstát, M.; Vajda, P. Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review. Nutrients 2020, 12, 2734. https://doi.org/10.3390/nu12092734
Hlinský T, Kumstát M, Vajda P. Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review. Nutrients. 2020; 12(9):2734. https://doi.org/10.3390/nu12092734
Chicago/Turabian StyleHlinský, Tomáš, Michal Kumstát, and Petr Vajda. 2020. "Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review" Nutrients 12, no. 9: 2734. https://doi.org/10.3390/nu12092734
APA StyleHlinský, T., Kumstát, M., & Vajda, P. (2020). Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review. Nutrients, 12(9), 2734. https://doi.org/10.3390/nu12092734






